See what API testing solution came out on top in the GigaOm Radar Report. Get your free analyst report >>


See what API testing solution came out on top in the GigaOm Radar Report. Get your free analyst report >>
Smiths Medical and Inovytec Automate Testing Through CI Pipeline Integration
Monrovia, CA – July 20, 2021 – Parasoft, a global leader in automated software testing for over 30 years, partnered with medical industry leaders, Smiths Medical and Inovytec, to help streamline the delivery of life-saving medical devices using its unified, fully integrated testing solution for C/C++ software development. Both innovative companies delivered safe, secure, and reliable products by implementing test automation practices into their development workflow.
Download the Inovytec and Smiths Medical success stories to find out how they delivered safe and secure embedded software.
When it comes to embedded software development, the medical device industry faces many challenges to reach compliance and deliver high-quality products. As a result, they must constantly evolve their development workflow. Some adjustments arise from customer requirement changes that lead to rework or redesign. Other changes stem from ever-changing suppliers, which can force changes to hardware and, in turn, the operating system, compiler, and development tools.
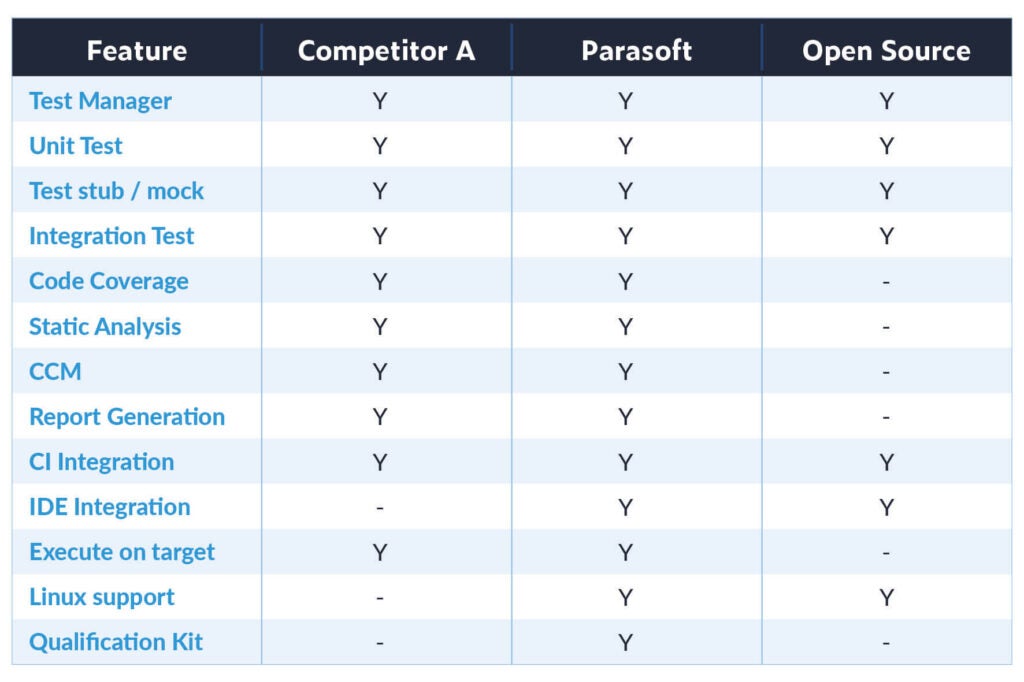
By automating the testing practices required by functional safety standards such as IEC 62304, medical organizations can reduce the cost and effort of achieving compliance. Parasoft solutions use AI and machine learning offering a unique approach to adopt static analysis.
Inovytec was able to customize C/C++ static code analysis rules to pass 100% of the FDA 510k certification rules and guidelines. “We not only noticed improvements in code quality, but C/C++test has really helped us in our static analysis verification activities and goal of achieving FDA 510(k) certification,” said Roi Birenshtok, solution architect and team leader of embedded software at Inovytec.
Parasoft understands the imperative need for medical device companies to constantly evolve and provides solutions to simplify testing processes for delivering compliant embedded software.
“One of the big areas that was a challenge in the past was the product learning curve. Tools that we had used in the past were too complicated to fit the needs of the team,” said Bill Schiller, senior principal software architect at Smiths Medical. “Parasoft C/C++test seamlessly integrates into our CI pipeline with a low learning curve, reducing adoption costs. We also wanted a partner with a market presence, which Parasoft has.”
Test automation is an important foundation of Smiths Medical’s testing approach. Schiller and his team wanted a solution to support their entire testing effort with a new approach and mindset of test-driven development (TDD). By choosing Parasoft C/C++test, they cut labor costs by 25%, increased code coverage to over 70%, and decreased code complexity to under 15 based on McCabe’s cyclomatic complexity measurements.
Watch the How Does Your Unit Testing Stack Up? on-demand webinar to hear a direct account of Smiths Medical’s test automation success story. Learn how your organization can reduce the cost of developing high-quality medical device software without sacrificing time to market.
About Parasoft
Parasoft helps organizations continuously deliver quality software with its market-proven, integrated suite of automated software testing tools. Supporting the embedded, enterprise, and IoT markets, Parasoft’s technologies reduce the time, effort, and cost of delivering secure, reliable, and compliant software by integrating everything from deep code analysis and unit testing to web UI and API testing, plus service virtualization and complete code coverage, into the delivery pipeline. Bringing all this together, Parasoft’s award winning reporting and analytics dashboard delivers a centralized view of quality enabling organizations to deliver with confidence and succeed in today’s most strategic ecosystems and development initiatives —security, safety-critical, Agile, DevOps, and continuous testing.
# # #
Press Contacts
For US inquiries:
Erika Delgado
+1 626 275 2434
For EMEA inquiries:
Beate Lorenzoni
+49 8122 559170